Chapter 3rd Metal And Non Metals For MP Super 100

Q1) Which of the following oxides of iron would be obtained on the prolonged reaction of iron with steam?
a) FeO
b) Fe2O3
c) Fe3O4
d) Fe2O3 and Fe3O4
Correct Answer: Option (c)
Q2) A non-metal used to preserve food material is:
a) Carbon
b) Phosphorus
c) Sulphur
d) Nitrogen
Correct Answer: Option (d)
Q3) The arrangement for Copper, Tin, Lead and Mercury, according to the reactivity series, is:
a) Tin> Lead> Copper> Mercury
b) Lead> Copper> Mercury> Tin
c) Copper> Mercury> Tin> Lead
d) Mercury> Tin> Lead> Copper
Correct Answer: Option (a)
Q4) The metals that float when treated with water are:
a) Manganese and sodium
b) Sodium and calcium
c) Magnesium and sodium
d) Magnesium and calcium
Correct Answer: Option (d)
Q5) Aluminium is used for making cooking utensils. Which of the following properties of aluminium are responsible for the same?
(i) Good thermal conductivity
(ii) Good electrical conductivity
(iii) Ductility
(iv) High melting point
a) (i) and (ii)
b) (i) and (iii)
c) (ii) and (iii)
d) (i) and (iv)
Correct Answer: Option (d)
Q6) When hydrochloric acid is added to barium hydroxide, a white-coloured compound is formed. Which of the following option gives the complete chemical reaction?
a) HCl + Ba(OH)2 → BaCl2 + 2HOH
b) 2HCl + Ba(OH)2 → BaCl2 + 2HOH
c) 2HCl + Ba(OH)2 → BaH2 + 2HCl + O2
d) HCl + 2Ba(OH) → 2BaCl2 + 2HOH + O2
Correct Answer: Option (b)
Q7) What happens when a pellet of sodium is dropped in water?
(a) It catches fire and forms oxide
(b) It absorbs heat and forms oxide
(c) It catches fire and forms hydroxide
(d) It absorbs heat and forms hydroxide
Correct Answer: Option (c)
Q8) The chemical reaction between a piece of copper and nitric acid is given by the chemical equations,
Cu + HNO3 → Cu(NO3)2 + H2
H2 + HNO3 → H2O + NO2
What can be inferred from the chemical equation?
a) Copper causes the oxidation of HNO3 to form NO2
b) Hydrogen gas gets oxidised by HNO3 to form water
c) Gas reacts with oxygen in the air to form water
d) Nitrate reacts with hydrogen to form NO2 and H2O
Correct Answer: Option (b)
Q9) Which of the following options gives the process of extraction of mercury from its ore cinnabar?
(a) Cooling cinnabar in the presence of excess air
(b) Cooling cinnabar to convert it into mercuric oxide and then heating it
(c) Cinnabar to convert it into mercuric oxide and then heating it again
(d) Cinnabar in the presence of limited air, and then adding a small amount of water
Correct Answer: Option (c)
Q10) The image shows the electrolytic refining of copper.
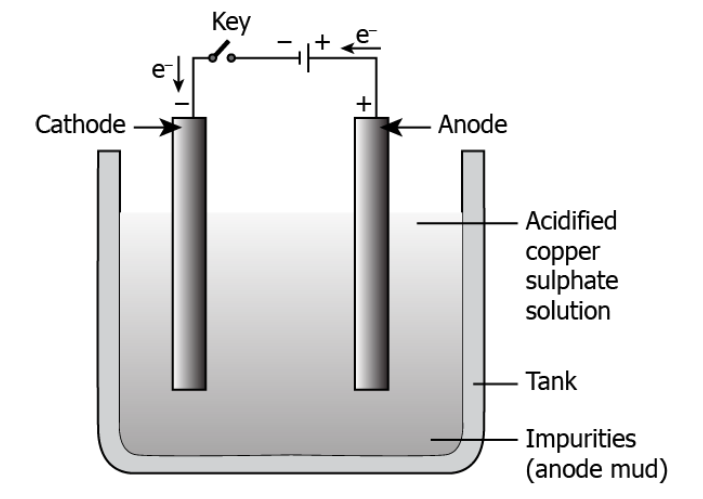
Which of the following options explains the process of obtaining pure copper?
(a) When current is passed, pure copper from the anode deposits to the cathode.
(b) When current is passed, pure copper from the anode deposits in the electrolytic solution.
(c) When current is passed, pure copper from the electrolytic solution deposits at the anode.
(d) When current is passed, pure copper from the electrolytic solution deposits at the cathode.
Correct Answer: Option (d)
Q11) When calcium oxide is added to water, it completely dissolves in water without forming bubbles. What products are formed in this reaction?
(a) Ca and H2
(b) Ca and H2O2
(c) Ca(OH)2
(d) CaH2
Correct Answer: Option (c)
Q12) A student studying the chemical properties of metals finds incomplete chemical reactions in his book, as shown below:
MgO + HNO3 →
Which option completes the reaction?
(a) MgO + HNO3 → Mg3N2 + 4H2O
(b) MgO + HNO3 → Mg + NO2 + O2
(c) MgO + HNO3 → Mg(OH)2 + 2NO2
(d) MgO + HNO3 → Mg(NO3)2 + H2O
Correct Answer: Option (d)
Q13) Which of the following can undergo a chemical reaction?
a) MgSO4 + Fe
b) ZnSO4 + Fe
c) MgSO4 + Pb
d) CuSO4 + Fe
Correct Answer: Option (d)
Q14) Pick the statement which is correct about the non-metal.
a) Br is an example of a liquid non-metal.
b) Graphite is a good conductor of electricity
c) Most of the non-metal oxides are acidic
d) All of these
Correct Answer: Option (d)
Q15) A student wrote two incomplete chemical reactions:

Which option completes the reactions to form a balanced chemical equation?
(a) X – P5O4(s); Y – (MgO)2(s)
(b) X – 4PO10(s); Y – 4MgO(s)
(c) X – P4O10(s); Y – 2MgO(s)
(d) X – 5P4O2(s); Y – Mg2O2(s)
Correct Answer: Option (c)
Q16) When a non-metal is allowed to react with water:
a) CO2 gas is formed
b) H2 gas is formed
c) Product formed depends on the temperature
d) No products are formed
Correct Answer: Option (d)
Q17) Aqua regia is a freshly prepared mixture of concentrated HNO3 and concentrated HCl in the ratio of:
a) 1:3, respectively
b) 2:3, respectively
c) 3:1, respectively
d) 3:2, respectively
Correct Answer: Option (a)








